
SPR curves of gold (circles), NTA alkanethiol on gold (triangles) and immobilized silicatetin (squares)
Proteins which induce or inhibit mineralization may be bound to surfaces with protein-engineered affinity tags, which leave the activity of the protein preserved. This has been achieved here with the protein silicatein, which has been reported to catalyze the formation of biosilicain order to study mineralization processes. The experimental results show that
- silicatein remains not only active but that the active site is oriented towards the solution
- silica formation occurs only at those parts of the surface where protein is bound
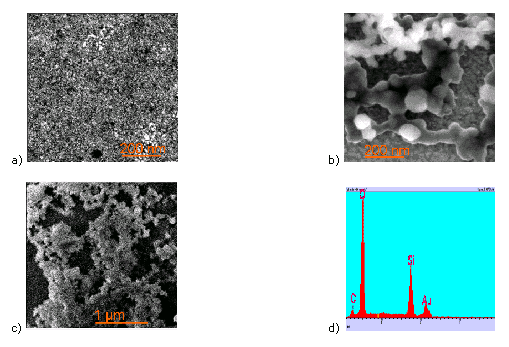
SEM images of silicatisation onto surfaces functionalised with NTA alkanethiol without Ni2+ (a) and NTA alkanethiol with Ni2+ chelating silicatein (b, c). No observable formation of SiO2 occured on NTA alkanethiol modified surfaces (a). In contast, Ni2+ chelated silicatein immobilization onto NTA alkanethiol surfaces induced the formation of SiO2 (b,c), as evidenced by the EDX-spectrum (d).

